Orphan Drugs Market – Japan
Market Statistics
Base Year: 2024
Historical Years: 2019-2024
Forecast Years: 2025-2033
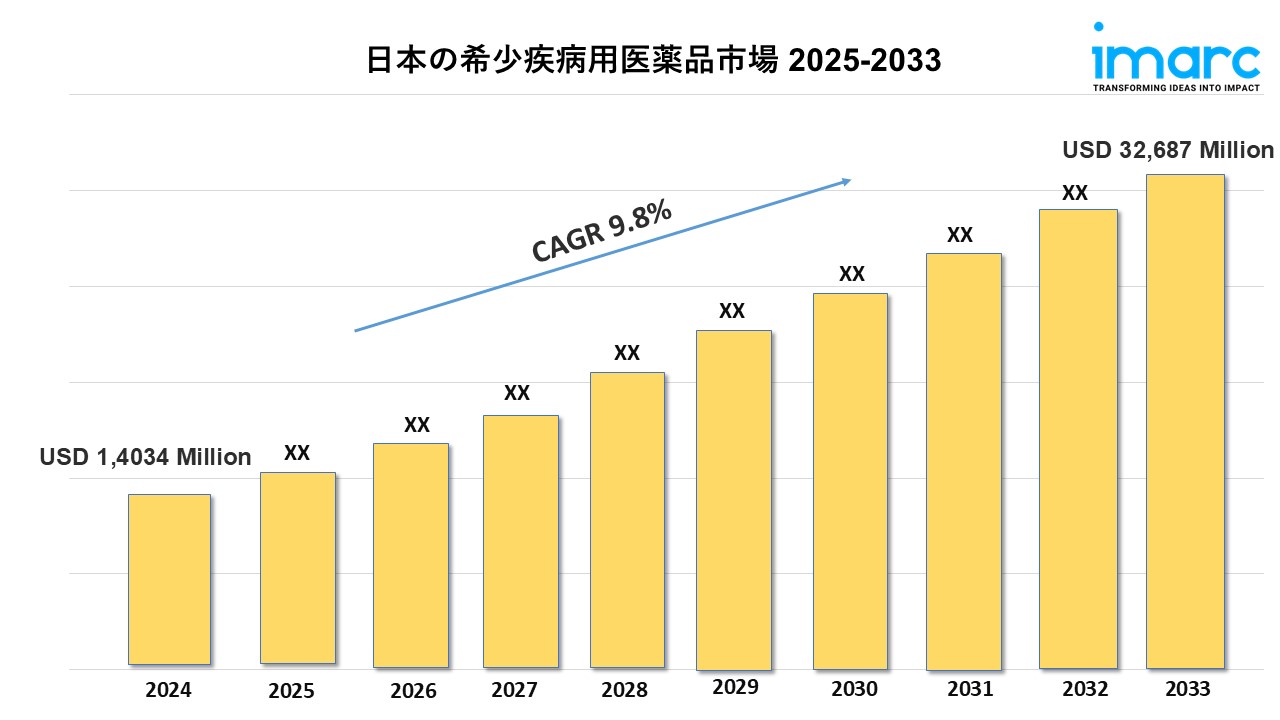
Market Size in 2024: USD 14,034 Million
Market Forecast in 2033: USD 32,687 Million
Market Growth Rate: 9.8% (2025-2033)
According to the latest report by IMARC Group, “Japan orphan drugs market size reached USD 14,034 Million in 2024. Looking forward, IMARC Group expects the market to reach USD 32,687 Million by 2033, exhibiting a growth rate (CAGR) of 9.8% during 2025-2033.”
Get Your Free Sample PDF Now: https://www.imarcgroup.com/japan-orphan-drugs-market/requestsample
Japan Orphan Drugs Industry Trends and Drivers:
The Japanese orphan drugs market is undergoing vigorous growth owing to the enabling nature of the regulatory environment in Japan as well as the rising awareness of unmet medical needs of patients with rare diseases. The Pharmaceuticals and Medical Devices Agency (PMDA) in Japan has adopted favorable policies that cover orphan drug designation, such as expedited reviews, longer market exclusivity and huge tax credits as an incentive to pharmaceutical companies to invest in rare disease research and development. The rising rate of diagnosis of rare diseases that previously were underdiagnosed due to the aging population of the country results in a high demand of specific treatments that mitigate these diseases. Also, the system of universal healthcare in Japan and the high reimbursement rates of orphan drugs guarantee patient access to such costly treatments, eliminating the financial barriers and promoting the market development of different types of rare diseases.
The development of technologies in precision medicine and genetic testing is transforming the process of identifying and treating rare diseases in Japan, allowing the application of a more precise diagnosis and treatment plan for the patient, which would positively affect patient-level outcomes and life quality. The increased partnership between Japanese pharmaceutical firms and foreign biotechnology is speeding up orphan drug development pipeline, introducing new therapies to the Japanese patients faster than ever in previous years. The robust research base and biotechnological experience in Japan and governmental programs aimed at the promotion of life sciences have made the country a promising market to developers of orphan drugs globally. In addition, efforts to raise patient awareness and advocacy are not only spurring better awareness about rare diseases among both healthcare providers and the general population, which is resulting in earlier diagnosis and improved compliance with treatment, but also is helping to promote further investment in orphan drug research and development in the oncology field and neurology among other fields.
Japan Orphan Drugs Market Segmentation:
The market report offers a comprehensive analysis of the segments, highlighting those with the largest Japan Orphan Drugs market share. It includes forecasts for the period 2025-2033 and historical data from 2019-2024 for the following segments.
Drug Type Insights:
- Biological
- Non-Biological
Disease Type Insights:
- Oncology
- Hematology
- Neurology
- Cardiovascular
- Others
Phase Insights:
- Phase I
- Phase II
- Phase III
- Phase IV
Top Selling Drugs Insights:
- Revlimid
- Rituxan
- Copaxone
- Opdivo
- Keytruda
- Imbruvica
- Avonex
- Sensipar
- Soliris
- Others
Distribution Channel Insights:
- Hospital Pharmacies
- Retail Pharmacies
- Online Stores
- Others
Regional Insights:
- Kanto Region
- Kinki Region
- Central/ Chubu Region
- Kyushu-Okinawa Region
- Tohoku Region
- Chugoku Region
- Hokkaido Region
- Shikoku Region
Request Customization for More Targeted Market Insights: https://www.imarcgroup.com/request?type=report&id=18562&flag=E
Competitive Landscape:
The report offers an in-depth examination of the competitive landscape. It includes a thorough competitive analysis encompassing market structure, key player positioning, leading strategies for success, a competitive dashboard, and a company evaluation quadrant. Additionally, the report features detailed profiles of all major companies in the Japan Orphan Drugs industry.
Key highlights of the Report:
- Market Performance (2019-2024)
- Market Outlook (2025-2033)
- COVID-19 Impact on the Market
- Porter’s Five Forces Analysis
- Strategic Recommendations
- Historical, Current and Future Market Trends
- Market Drivers and Success Factors
- SWOT Analysis
- Structure of the Market
- Value Chain Analysis
- Comprehensive Mapping of the Competitive Landscape
Note: If you need specific information that is not currently within the scope of the report, we will provide it to you as a part of the customization.
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact:
Street: 563-13 Kamien
Area: Iwata
Country: Tokyo Japan
Postal Code: 4380111
Email: sales@imarcgroup.com